

How To Reduce Total Dissolved Solids (TDS) In Water?
The ways to reduce Total Dissolved Solids (TDS) in water are reverse osmosis (most common process, 90-99% removal of dissolved solids using a semi-permeable membrane),
# Type at least 1 character to search # Hit enter to search or ESC to close

No products in the cart.

No products in the cart.
Product Categories

Dissolved oxygen (DO) plays a crucial role in groundwater quality. It supports bacteria that break down pollutants and minimizes harmful substances like iron and manganese. High levels of dissolved oxygen help maintain low contaminant levels, leading to cleaner, safer water. Conversely, low dissolved oxygen can lead to the accumulation of toxins, impacting both drinking water and surrounding ecosystems. Regular monitoring is essential for sustaining good water quality.
Groundwater requires dissolved oxygen much like humans need air to breathe. This invisible but essential component dictates groundwater health, influencing everything from water chemistry to the survival of aquatic organisms.
Dissolved oxygen levels vary significantly across different aquifers, revealing insights about groundwater conditions. These variations, typically ranging from 0-10 mg/L, impact biological and chemical processes in the subsurface environment.
Dissolved oxygen refers to the free oxygen molecules available in water. Unlike the oxygen bound within H₂O molecules, dissolved oxygen is accessible to aquatic organisms.
Typically measured in milligrams per liter (mg/L) or as a percentage of saturation, healthy groundwater contains between 0-10 mg/L of dissolved oxygen, depending on local conditions.
The presence of dissolved oxygen (DO) in groundwater largely depends on the depth of the aquifers from which the water is sourced.
As water percolates downward through the soil, it initially comes into contact with the atmosphere, allowing oxygen to dissolve into the water. However, the concentration of dissolved oxygen can vary depending on the depth of the aquifer and the conditions within it.

In deeper aquifers, DO can still be present as long as there is little or no oxidizable material, which would otherwise consume the oxygen. In such cases, oxygen can remain in groundwater at significant depths, sustaining a balance in the water chemistry and supporting subsurface ecosystems.
Groundwater receives oxygen from several sources, including:

These sources are balanced by “sinks” that consume oxygen. These include microbial respiration, chemical oxidation reactions, and the breakdown of organic matter. The balance between these sources and sinks determines dissolved oxygen levels in groundwater.
Several factors can influence the amount of dissolved oxygen in groundwater:
By understanding these factors, better management of groundwater resources can be achieved, aiding in the prediction of environmental changes and their impact on subsurface ecosystems.
Accurately measuring dissolved oxygen is essential for understanding groundwater quality. Technological advancements have made it possible to assess dissolved oxygen with precision using specialized equipment.
Field sampling provides the most accurate picture of groundwater conditions, as dissolved oxygen levels can change rapidly when groundwater is exposed to air. Specialized tools, like biochemical oxygen demand (BOD) bottles, are employed to take precise samples without trapping air bubbles. Field measurements often include:

Laboratory analysis offers a more controlled environment for dissolved oxygen measurement. The Winkler Method, a traditional chemical technique, requires careful titration to determine oxygen levels. Today, modern technology has introduced electrochemical sensors and optical dissolved oxygen meters, which provide accurate and quick readings.
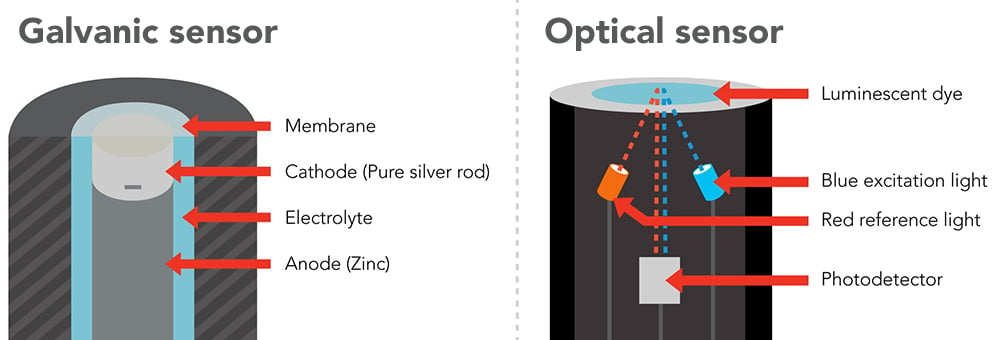
Atlas Scientific’s electrochemical dissolved oxygen probes are engineered to deliver precise readings, even in challenging environments. Our probes are designed with durability in mind, featuring robust materials that withstand harsh field conditions while maintaining consistent accuracy. Our commitment to precision ensures our probes are a top choice for professionals who need dependable data to guide environmental management.
At Atlas Scientific, we have established ourselves as a trusted provider of high-quality dissolved oxygen probes, tailored to diverse needs. Our sensors are known for:
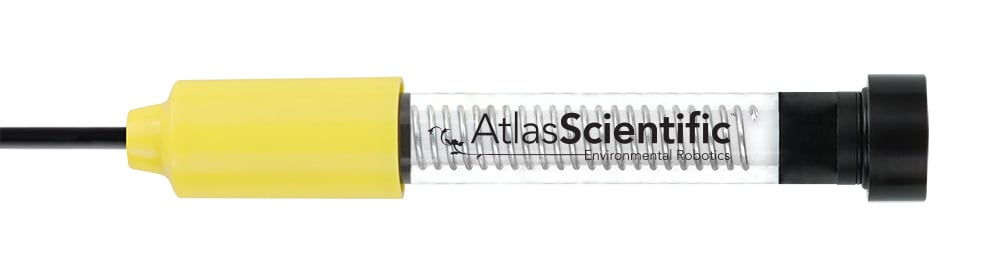
By incorporating Atlas Scientific’s dissolved oxygen probes into groundwater monitoring efforts, environmental managers and scientists can better understand dissolved oxygen dynamics, identify potential contamination risks, and develop effective strategies for groundwater quality improvement. The reliable performance of these probes supports accurate and informed decision-making, ultimately contributing to healthier ecosystems and safer drinking water supplies.
Quality control is crucial in ensuring reliable results, including:
Maintaining the integrity of samples is essential; temperature control and limiting exposure to air are particularly important. Calibration of dissolved oxygen sensors against known standards is also a routine part of quality assurance.
Dissolved oxygen shapes groundwater chemistry through various processes, influencing both water quality and ecosystem health.
Dissolved oxygen acts as a primary electron acceptor in groundwater systems, promoting a series of chemical reactions. High dissolved oxygen levels (above 1 mg/L) create oxidizing conditions that lead to:
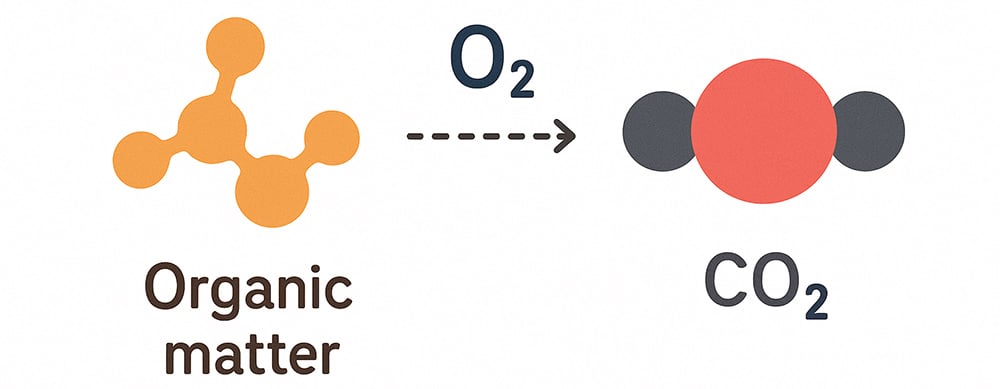
These reactions significantly affect groundwater composition, influencing everything from metal solubility to the presence of nutrients.
Dissolved oxygen plays a pivotal role in the behavior of metals within groundwater. High dissolved oxygen concentrations can stabilize certain metals by forming insoluble oxides, while low-oxygen conditions (below 0.5 mg/L) may cause metals like iron and manganese to become more mobile, potentially leading to contamination.
Changes in oxygen levels can also release metals previously trapped in sediments, a phenomenon known as “secondary contamination.” This underscores the importance of dissolved oxygen in predicting and managing groundwater quality.
Microbial activity is highly dependent on dissolved oxygen levels. In oxygen-rich environments, microorganisms use dissolved oxygen as an electron acceptor to break down organic pollutants. When dissolved oxygen is scarce, microbes shift to alternative acceptors like nitrate, manganese, iron, and sulfate.

This sequence of reactions affects groundwater composition, with specific dissolved oxygen levels supporting distinct microbial communities.
Dissolved oxygen not only influences groundwater chemistry but also impacts the health of subsurface ecosystems.
Dissolved oxygen is a crucial indicator of habitat health for aquatic species. Key thresholds for groundwater ecosystems are:
Fluctuating DO levels create dynamic environments, influencing which species can thrive. More adaptable organisms may survive lower DO, while sensitive species might relocate or perish.
Microbial communities, the foundation of groundwater ecosystems, are highly sensitive to dissolved oxygen. Their activity directly impacts water quality through processes like nutrient cycling and organic matter decomposition. Seasonal variations, particularly temperature shifts, can alter microbial dynamics by affecting oxygen demand.
Dissolved oxygen is a vital component of groundwater health, influencing everything from water chemistry to the survival of aquatic habitats. Adequate dissolved oxygen levels are crucial for maintaining clean water and supporting the delicate balance of subsurface ecosystems.

Regular monitoring and effective management of dissolved oxygen levels are essential to preserving groundwater quality. Accurate measurement techniques and stringent quality control are vital in protecting these ecosystems. By understanding the influence of dissolved oxygen on groundwater chemistry, potential water quality challenges can be anticipated and effectively addressed.
If you would like to learn more about the importance of dissolved oxygen, or what dissolved oxygen sensors we offer, do not hesitate to contact the world-class team at Atlas Scientific.

The ways to reduce Total Dissolved Solids (TDS) in water are reverse osmosis (most common process, 90-99% removal of dissolved solids using a semi-permeable membrane),

Finding the best pH probe for hydroponics can make or break your growing success. In hydroponics, maintaining the correct pH is essential because it directly
Notifications